Abstract
Background: Bortezomib, lenalidomide, dexamethasone (VRd) and carfilzomib, lenalidomide, dexamethasone (KRd) are standard induction regimens for the treatment of newly diagnosed multiple myeloma (NDMM). The phase III ENDURANCE trial excluded patients with high-risk NDMM (HR-NDMM) and included patients with no intention for immediate autologous stem cell transplant (ASCT) (Kumar SK, et al. Lancet Oncol 2020). Herein, we examined outcomes associated with KRd and VRd induction in the management of HR-NDMM.
Methods: We conducted a retrospective chart review study with 154 consecutive HR-NDMM patients treated with VRd and KRd at MSKCC between 1/1/2015 to 12/31/2019. Only patients with high-risk (HR) cytogenetics defined as 1q+, t(4;14), t(14;16), t(14;20), and/or del(17p) were included. The cutoff date for analysis was 8/18/21. Early transplant was defined as ASCT within 6 months of completing induction. Primary endpoint was progression-free survival (PFS). Discrete patient characteristics were summarized by frequency (percentage) and continuous characteristics were summarized by median (IQR). PFS and overall survival (OS) were evaluated by Kaplan-Meier method. Multivariable Cox models were fitted with pre-specified clinical parameters including age, cardiac history, R-ISS stage, best response to induction, early ASCT (both time dependent covariates), and induction regimen.
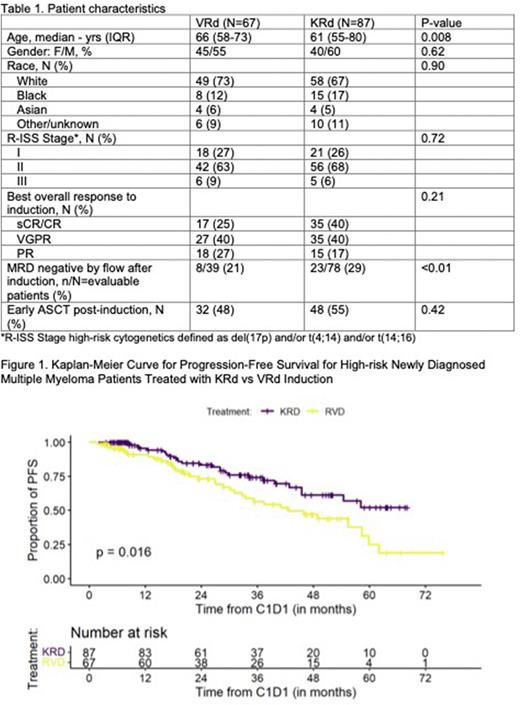
Results: Baseline characteristics of the 67 VRd and 87 KRd treated patients are summarized in table 1. There were 51 (76%) patients in the VRd group and 63 (72%) patients in the KRd group with 1 HR cytogenetic abnormality. Sixteen (24%) and 24 (28%) patients in the VRd and KRd groups, respectively, had ≥2 HR cytogenetic abnormalities. Among the HR-NDMM patients, 32 VRd- and 48 KRd-treated patients received early ASCT. Overall response rate by the end of induction was 93% and 98% for VRd and KRd, respectively, including 66% with ≥VGPR in the VRd group and 80% in the KRd group. Median follow-up for the entire patient population was 42.5 (IQR, 37.5-50) months with 47.9 (42.5-53.9) months for the VRd group and 37.6 (35-50) months for the KRd group. The median PFS for HR-NDMM patients treated with VRd induction was 42.6 months (95%CI, 32.8-62) and was NR (95%CI, 45.5-NR) for the KRd group (HR=1.84; 95%CI, 1.11-3.06; P=0.02) (Fig. 1).
We conducted a multivariate analysis for important clinical variables that may affect survival outcomes. Multivariable analysis for PFS showed that KRd induction (HR=1.80; 95%CI, 1.05-3.10; P=0.033) and R-ISS Stage I compared to R-ISS Stage II (HR=2.67; 95%CI, 1.33-5.38; P=0.006) and R-ISS Stage III (HR=3.51; 95%CI, 1.02-12.07; P=0.046) were associated with better PFS. Early ASCT was not associated with improvement of PFS on multivariable analysis (P=0.30). Age, cardiac history, and achieving CR/sCR or VGPR/PR compared to <PR as the best response to induction did not have a statistically significant effect on PFS.
We conducted a subgroup analysis of HR-NDMM patients who received early ASCT (N=79). At median follow-up since time of transplant of 43.5 months for VRd and 28.4 months for KRd, the estimated 3-year PFS since the time of transplant was 55% (95%CI, 38-79) and 67% (95%CI, 52-85) for VRd and KRd-treated patients, respectively (HR=1.65; 95%CI, 0.78-3.50; P=0.20). The estimated 3-year OS from the time of transplant was 80% (95%CI, 66-81) for the VRd group and 91% (95%CI, 81-100) for the KRd group, respectively (HR=2.45; 95%CI, 0.60-10.0; P=0.20).
Conclusion: There was a significant improvement in PFS among HR-NDMM patients who received KRd compared to VRd induction. In patients who received early ASCT, we did not observe a statistically significant difference in PFS or OS between the two induction regimens, but this analysis is subject to inherent selection bias. Until results from prospective randomized studies are available for HR-NDMM patients, the use of various induction regimens in transplant-eligible patients opting for early transplant should be tailored to each patient.
Disclosures
Tan:Janssen: Honoraria, Research Funding. Hultcrantz:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Curio Science LLC: Consultancy; Amgen, Daichii Sankyo, Cosette, GSK: Research Funding; Intellisphere LLC: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hassoun:Janssen Pharmaceuticals: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Mailankody:Fate Therapeutics: Research Funding; Plexus Communication: Honoraria; Bristol Myers Squibb: Research Funding; Juno Therapeutics: Research Funding; Physician Education Resource: Honoraria; OncLive: Honoraria; Takeda Oncology: Research Funding; Allogene Therapeutics: Research Funding; Optum Oncology: Consultancy; BioAscend: Consultancy; Janssen Oncology: Consultancy, Research Funding; Evicore: Consultancy; Legend Biotech: Consultancy; Memorial Sloan Kettering Cancer Center: Current Employment. Shah:MJH Lifesciences: Consultancy, Honoraria; ACCC: Honoraria; MashUpMD: Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Sanofi: Consultancy. Lahoud:MorphoSys, Inc: Membership on an entity's Board of Directors or advisory committees. Shah:Beyond Spring: Research Funding; Janssen: Research Funding; Amgen: Research Funding. Scordo:Angiocrine Bioscience, Inc.: Consultancy, Research Funding; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); McKinsey & Company: Consultancy; i3Health (CME): Honoraria; Medscape, LCC (CME): Honoraria. Landau:Janssen: Consultancy; Takeda Pharmaceuticals: Consultancy, Other: grants/pending grants; Legend Biotech USA Inc: Consultancy; Caelum Biosciences: Consultancy; Juno: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Karyopharm: Consultancy; Prothena: Honoraria; Memorial Sloan Kettering Cancer Center: Current Employment; Alexion: Other: grants/pending grants; Janssen Scientific Affairs, LLC: Other: grants/pending grants. Giralt:Omeros: Research Funding; Takeda: Consultancy, Research Funding; Miltenyi: Research Funding; Spectrum Pharma: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Jazz Pharmaceutical: Consultancy; Celgene: Consultancy, Research Funding; Johnson & Johnson: Consultancy, Research Funding; Kite: Consultancy; Actinuum: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Lesokhin:Sanofi: Research Funding; Trillium Therapeutics: Consultancy, Research Funding; Janssen, Pfizer, Iteos, Sanofi, Genmab: Honoraria; Amgen: Honoraria; BMS: Honoraria; Serametrix, inc: Patents & Royalties; Pfizer, Genmab, Sanofi, Iteos, BMS, Janssen: Consultancy; Janssen, Pfizer, BMS, Genentech/Roche: Research Funding; Memorial Sloan Kettering Cancer Center: Current Employment. Korde:Clinical Care Options, OncLive, Intellisphere: Consultancy; Amgen, Janssen: Research Funding. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal